Validation of Clinical Development and Pricing Strategy
Background
A German-based biotech in discussion with a Japanese company about the potential licensing of its product. The product targets the treatment of a disease with large unmet medical need currently receiving little treatment.
The product was finishing its Phase III program in Europe with plans to submit the application for approval to the authorities.
Objective
As the two companies were negotiating the deal terms, the German client (the potential licensor) sought an objective perspective on the commercial potential of the product.
e-Projection carried out research to provide more clarity on the prospects of the product in Japan.
Project Summary
-
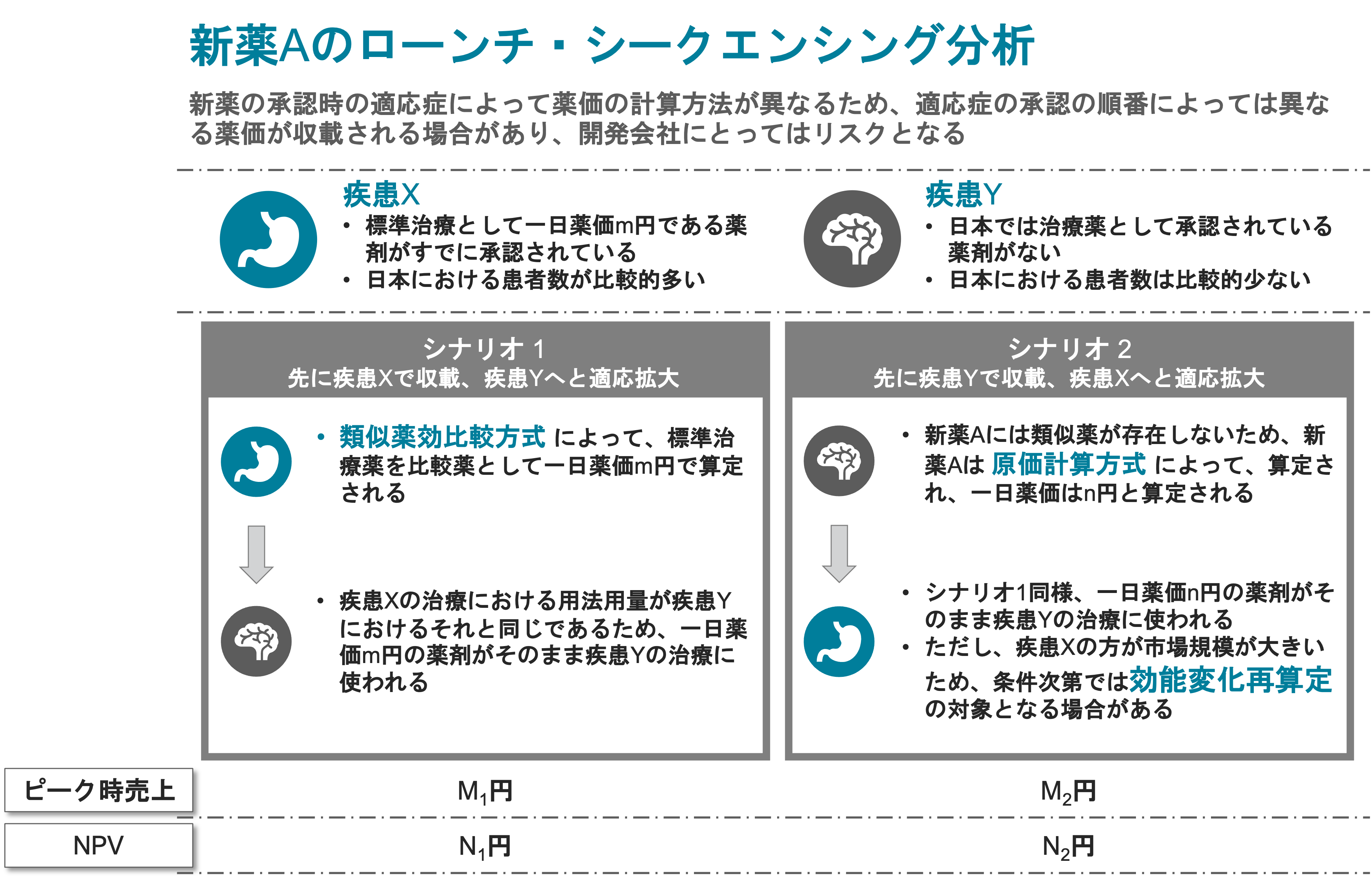
Assessed development scenarios based on available data gathered in EU (USA).
Assessment conducted with regard to the requirements of the Japanese authority to commercialize the product in Japan. - Multiple possible pricing scenarios were listed, along with the rationale behind each scenario.
- Identified the effect of variables on the pricing decision.
- Analyzed market data and projected demand for drugs in the same category.
Client Feedback
For the market report, e-Projection assessed the scientific rationale behind each of the lead products, the clinical data collected to date and the suggested clinical development plans.
We used this information to create an independent report proposing feasible pricing in each indication and the available patient population, market share and market penetration.
Timeline
8 weeks
Key Project Capabilities
- Pricing and Market Access Consultation
- Regulatory Consultation
- Clinical Consultation
- Demand Analysis
- Market Research
Deliverables
- Excel-based forecasting model
Population-based
Multiple scenarios
Parametric
Reflects competitive landscape
Evidence/fact-based time series analysis using cutting edge algorithms - PowerPoint presentation
Suggestions for clinical development strategy
Potential pricing scenarios and estimated price dynamics
We help non-Japanese pharma/biotech companies
understand and expand into the Japanese market.