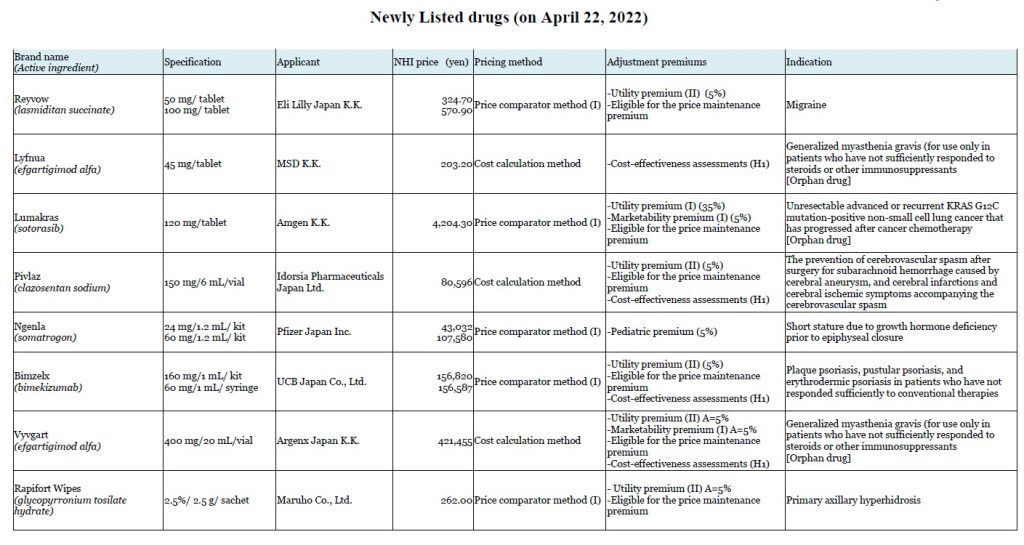
Chuikyo approved listing of 8 Drugs on April 20
8 new drugs will join the reimbursement price list in Japan on April 20.
In the FY2022 Drug pricing reform, the Chuikyo introduced “the premium coefficient 0 (less than 50% degree of disclosure)”, which is a new rule to improve transparency of manufacturing costs used in the cost calculation method for newly priced products, particularly products imported overseas. Vyvgart, a treatment for generalized myasthenia gravis, and Pivlaz, the prevention of cerebral vasospasms, have become the first two drugs that this rule was applied to. Both Vyvgart and Pivlaz have received some sort of the premiums, but none of these were reflected in their NHI prices because the premium coefficient 0 (less than 50% degree of disclosure) was applied.
Four of the newly listed drugs carry peak sales forecasts topping 10 billion yen, including Vyvgart and Pivlaz.
Contact us if you have any questions about the Japanese pricing system.